Merck Anti-Tau-1 Antibody, clone PC1C6
✨AI 추천 연관 상품
AI가 분석한 이 상품과 연관된 추천 상품들을 확인해보세요
연관 상품을 찾고 있습니다...
Anti-Tau-1 Antibody, clone PC1C6
clone PC1C6, Chemicon®, from mouse
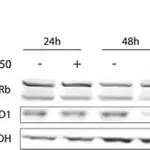
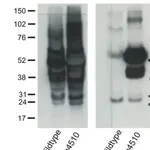
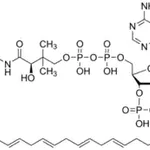
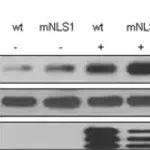
Tau, a microtubulebinding protein which serves to stabilize microtubules in growing axons, is found to be hyperphosphorylated in paired helical filaments (PHF), the major fibrous component of neurofibrillary lesions associated with Alzheimer’s disease. Hyperphosphorylation of Tau is thought to be the critical event leading to the assembly of PHF. Six Tau protein isoforms have been identified, all of which are phosphorylated by glycogen synthase kinase 3 (GSK 3). Cellular and subcellular localization: In situ, anti-tau-1 has a stringent specificity for the axons of neurons. The antibody does not stain the cell bodies or dendrites of neurons, nor does it stain any other cell type (4). However, this in vivo intracellular specificity is not maintained in culture: anti-tau-1 stains the axon, cell bodies, and dendrites of rat hippocampal neurons grown in culture (5). The specificity of anti-tau-1 was originally thought to represent the restricted expression of tau to axons. Later studies revealed that this specificity is dependant on the state of phosphorylation. In dephosphorylated samples (samples treated with alkaline phosphatase) anti-tau-1 stains astrocytes, perineuronal glial cells, and the axons, cell bodies and dendrites of neurons, while in untreated samples, anti-tau-1 stains only axons (6). (The epitope recognized by anti-tau-1 is probably at or near a phosphorylated site.)
🏷️Merck Sigma 상품 둘러보기
동일 브랜드의 다른 상품들을 확인해보세요
배송/결제/교환/반품 안내
배송 정보
| 기본 배송비 |
| 교환/반품 배송비 |
|
|---|---|---|---|
| 착불 배송비 |
| ||
| 교환/반품 배송비 |
| ||
결제 및 환불 안내
| 결제수단 |
|
|---|---|
| 취소 |
|
| 반품 |
|
| 환급 |
|
교환 및 반품 접수
| 교환 및 반품 접수 기한 |
|
|---|---|
| 교환 및 반품 접수가 가능한 경우 |
|
| 교환 및 반품 접수가 불가능한 경우 |
|
교환 및 반품 신청
| 교환 절차 |
|
|---|---|
| 반품 절차 |
|