
Merck Anti-GAD67 Antibody, clone 1G10.2
✨AI 추천 연관 상품
AI가 분석한 이 상품과 연관된 추천 상품들을 확인해보세요
연관 상품을 찾고 있습니다...
Anti-GAD67 Antibody, clone 1G10.2
clone 1G10.2, Chemicon®, from mouse
Glutamate Decarboxylase 67kDa Isoform, Glutamate Decarboxylase 1, Glutamic acid decarboxylase 1
Gutamic acid decarboxylase (GAD; E.C. 4.1.1.15) is the enzyme responsible for the conversion of glutamic acid to gamma-aminobutyric acid (GABA), the major inhibitory transmitter in higher brain regions, and putative paracrine hormone in pancreatic islets. Two molecular forms of GAD (65 kDa and 67 kDa, 64% aa identity between forms) are highly conserved and both forms are expressed in the CNS, pancreatic islet cells, testis, oviduct and ovary. The isoforms are regionally distributed cytoplasmically in the brains of rats and mice (Sheikh, 1999). GAD65 is an amphiphilic, membrane-anchored protein (585 a.a.), encoded on human chromosome 10, and is responsible for vesicular GABA production. GAD67 is cytoplasmic (594 a.a.), encoded on chromosome 2, and seems to be responsible for significant cytoplasmic GABA production. GAD expression changes during neural development in rat spinal cord. GAD65 is expressed transiently in commissural axons around E13 but is down regulated the next day while GAD67 expression increases mostly in the somata of those neurons (Phelps, 1999). In mature rat pancreas, GAD65 and GAD67 appear to be differentially localized, GAD65 primarily in insulin-containing beta cells and GAD67 in glucagon-containing (A) cells (Li, 1995). GAD67 expression seems to be particularly plastic and can change in response to experimental manipulation (for example neuronal stimulation or transection) or disease progression and emergent disorders like schizophrenia (Volk, 2000). Colocalization of the two GAD isoforms also shows changes in GAD65/GAD67 distributions correlated with certain disease states such as IDDM and SMS.
🏷️Merck Sigma 상품 둘러보기
동일 브랜드의 다른 상품들을 확인해보세요
Merck Sigma
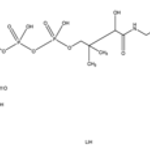
Merck β-Methylcrotonyl coenzyme A lithium salt
645,500원
Merck Sigma
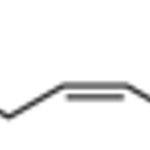
Merck cis-5,8,11-Eicosatrienoic acid methyl ester
339,400원
Merck Sigma
Merck Anti-GAD67 Antibody, clone 1G10.2
206,000원
Merck Sigma
Merck Anti-Glial Fibrillary Acidic Protein Antibody, clone GA5
205,900원
Merck Sigma
Merck Anti-Sox9 Antibody
206,000원
배송/결제/교환/반품 안내
배송 정보
| 기본 배송비 |
| 교환/반품 배송비 |
|
|---|---|---|---|
| 착불 배송비 |
| ||
| 교환/반품 배송비 |
| ||
결제 및 환불 안내
| 결제수단 |
|
|---|---|
| 취소 |
|
| 반품 |
|
| 환급 |
|
교환 및 반품 접수
| 교환 및 반품 접수 기한 |
|
|---|---|
| 교환 및 반품 접수가 가능한 경우 |
|
| 교환 및 반품 접수가 불가능한 경우 |
|
교환 및 반품 신청
| 교환 절차 |
|
|---|---|
| 반품 절차 |
|
